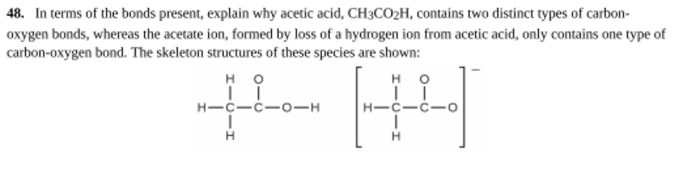
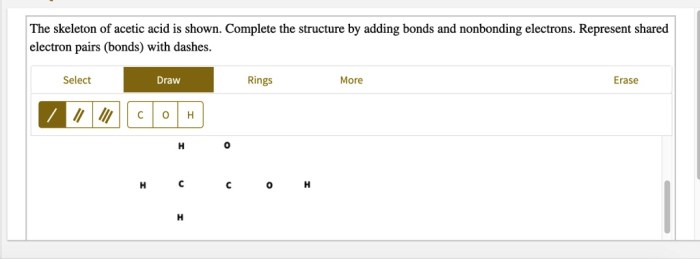
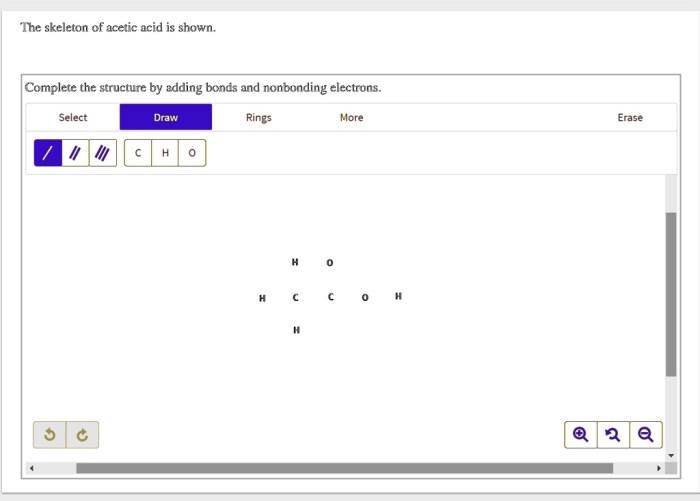
The skeleton of acetic acid is shown, revealing the fundamental structure of this ubiquitous organic compound. Understanding its molecular geometry and bonding characteristics is crucial for comprehending its chemical behavior and diverse applications.
The tetrahedral geometry of the carbon atom, the hybridization of its orbitals, and the polarity of its bonds shape the molecular interactions and reactivity of acetic acid.
Introduction
The acetic acid skeleton, consisting of a central carbon atom bonded to three hydrogen atoms and a carboxylic acid group (-COOH), serves as a fundamental structure in organic chemistry. Understanding its molecular geometry, bonding characteristics, and functional group reactivity is crucial for comprehending its chemical behavior and applications.
Molecular Geometry and Bonding
The carbon atom in the acetic acid skeleton adopts a tetrahedral geometry, with bond angles of approximately 109.5 degrees. This geometry results from the sp 3hybridization of the carbon atom, which involves the promotion of one 2s electron to a 2p orbital, leading to the formation of four equivalent sp 3hybrid orbitals.
The four sp 3hybrid orbitals overlap with the 1s orbitals of the three hydrogen atoms and the 2p orbital of the oxygen atom in the carboxylic acid group, forming four sigma (σ) bonds.
Functional Groups
The acetic acid skeleton contains two important functional groups: the carboxylic acid group (-COOH) and the methyl group (-CH 3). The carboxylic acid group consists of a carbonyl group (C=O) and a hydroxyl group (-OH), and it imparts acidic properties to acetic acid.
The methyl group is a saturated hydrocarbon group that provides a nonpolar character to the molecule.
Resonance and Tautomerism, The skeleton of acetic acid is shown
The acetic acid skeleton exhibits resonance, which involves the delocalization of electrons within the molecule. The resonance structures of acetic acid show that the double bond between the carbon and oxygen atoms in the carbonyl group can be represented as a single bond with a negative charge on the oxygen atom and a positive charge on the carbon atom.
This resonance contributes to the stability of acetic acid and influences its chemical reactivity. Additionally, acetic acid can undergo tautomerism, a reversible isomerization process that involves the transfer of a proton between the hydroxyl group and the carbonyl oxygen, resulting in the formation of the enol tautomer.
Spectroscopic Analysis
The acetic acid skeleton can be identified and characterized using various spectroscopic techniques. Infrared (IR) spectroscopy shows a characteristic absorption band at around 1710 cm -1due to the C=O stretching vibration. Nuclear magnetic resonance (NMR) spectroscopy reveals signals corresponding to the methyl protons (δ ~ 2 ppm), the methylene protons (δ ~ 1.9 ppm), and the carboxylic acid proton (δ ~ 12 ppm).
Mass spectrometry can be used to determine the molecular weight and elemental composition of acetic acid.
Applications
Acetic acid has a wide range of applications, including:
- Food preservation: As vinegar, acetic acid is used as a preservative in food items such as pickles, sauces, and condiments.
- Textile manufacturing: Acetic acid is used in the production of synthetic fibers such as rayon and acetate.
- Chemical synthesis: Acetic acid is a versatile starting material for various chemical reactions, including the synthesis of pharmaceuticals, dyes, and plastics.
Clarifying Questions: The Skeleton Of Acetic Acid Is Shown
What is the significance of the acetic acid skeleton?
The acetic acid skeleton is a fundamental structure in organic chemistry, serving as the backbone of numerous compounds and playing a crucial role in various chemical processes.
How does the molecular geometry of acetic acid influence its properties?
The tetrahedral geometry of the carbon atom in acetic acid determines its bond angles and lengths, affecting its polarity and molecular interactions, which in turn influence its reactivity and chemical behavior.
What functional groups are present in the acetic acid skeleton, and how do they contribute to its reactivity?
The acetic acid skeleton contains a carboxylic acid group (-COOH), which is responsible for its characteristic acidity and reactivity. This functional group undergoes various reactions, including esterification, amidation, and halogenation.