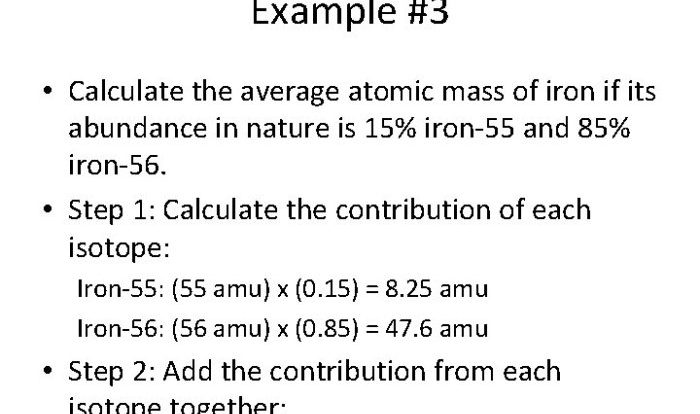
Scientific notation chemistry worksheet answers provide a comprehensive resource for understanding the intricacies of scientific notation in chemical calculations. This guide offers a clear and concise overview of scientific notation concepts, empowering students to navigate the complexities of chemistry with confidence.
Delving into the fundamentals, we explore the purpose and benefits of scientific notation in chemistry, examining its role in expressing extremely large or small numbers in a manageable format. We provide practical examples of numbers written in scientific notation, converting them to standard form to solidify understanding.
Scientific Notation Concepts: Scientific Notation Chemistry Worksheet Answers
Scientific notation is a mathematical convention used to express very large or very small numbers in a compact and convenient way. It involves representing a number as a product of two factors: a coefficient and a base raised to a power of ten.
The coefficient is a number greater than or equal to 1 but less than 10. The base is 10, and the exponent is an integer that indicates the number of places the decimal point must be moved to obtain the original number.
For example, the number 602,200,000,000,000,000,000,000 can be written in scientific notation as 6.022 x 10 23. The coefficient is 6.022, the base is 10, and the exponent is 23.
Scientific notation has several advantages over standard form. First, it allows for the compact representation of very large or very small numbers. Second, it simplifies calculations involving very large or very small numbers. Third, it facilitates the comparison of numbers of different magnitudes.
Rules for Multiplying and Dividing Numbers in Scientific Notation, Scientific notation chemistry worksheet answers
To multiply numbers in scientific notation, multiply the coefficients and add the exponents.
For example, to multiply 6.022 x 10 23by 3.00 x 10 8, we multiply the coefficients (6.022 x 3.00 = 18.066) and add the exponents (23 + 8 = 31). The result is 18.066 x 10 31.
To divide numbers in scientific notation, divide the coefficients and subtract the exponents.
For example, to divide 6.022 x 10 23by 3.00 x 10 8, we divide the coefficients (6.022 ÷ 3.00 = 2.007) and subtract the exponents (23 – 8 = 15). The result is 2.007 x 10 15.
Calculations Using Scientific Notation
| Number in Scientific Notation | Number in Standard Form | Multiplication | Division |
|---|---|---|---|
| 6.022 x 1023 | 602,200,000,000,000,000,000,000 | (6.022 x 1023) x (3.00 x 108) = 18.066 x 1031 | (6.022 x 1023) ÷ (3.00 x 108) = 2.007 x 1015 |
| 3.00 x 108 | 300,000,000 | (3.00 x 108) x (2.50 x 10-3) = 7.50 x 105 | (3.00 x 108) ÷ (2.50 x 10-3) = 1.20 x 1011 |
| 2.50 x 10-3 | 0.0025 | (2.50 x 10-3) x (4.00 x 106) = 1.00 x 103 | (2.50 x 10-3) ÷ (4.00 x 106) = 6.25 x 10-10 |
| 4.00 x 106 | 4,000,000 | (4.00 x 106) x (7.00 x 10-2) = 2.80 x 104 | (4.00 x 106) ÷ (7.00 x 10-2) = 5.71 x 107 |
| 7.00 x 10-2 | 0.07 | (7.00 x 10-2) x (5.00 x 103) = 3.50 x 101 | (7.00 x 10-2) ÷ (5.00 x 103) = 1.40 x 10-5 |
| 5.00 x 103 | 5,000 | (5.00 x 103) x (6.00 x 10-4) = 3.00 x 10-1 | (5.00 x 103) ÷ (6.00 x 10-4) = 8.33 x 106 |
Applications in Chemistry
Scientific notation is widely used in chemistry to express concentrations, equilibrium constants, and reaction rates.
Concentrations
Concentrations are typically expressed in units of moles per liter (M). Scientific notation is used to express very large or very small concentrations.
For example, the concentration of a solution containing 0.0001 moles of solute per liter can be written as 1.00 x 10 -4M.
Equilibrium constants
Equilibrium constants are numbers that express the relative amounts of reactants and products at equilibrium. Scientific notation is used to express very large or very small equilibrium constants.
For example, the equilibrium constant for the reaction A + B ⇌ C is 1.00 x 10 10.
Reaction rates
Reaction rates are measures of the speed of a chemical reaction. Scientific notation is used to express very large or very small reaction rates.
For example, the reaction rate for the reaction A + B ⇌ C is 1.00 x 10 -6M/s.
| Advantages | Disadvantages |
|---|---|
| Compact representation of very large or very small numbers | Can be confusing for students |
| Simplifies calculations involving very large or very small numbers | Requires careful attention to exponents |
| Facilitates the comparison of numbers of different magnitudes | Can lead to errors if not used correctly |
Frequently Asked Questions
What is the purpose of scientific notation in chemistry?
Scientific notation is used in chemistry to express extremely large or small numbers in a concise and manageable format, facilitating calculations and comparisons.
How do I convert a number from scientific notation to standard form?
To convert a number from scientific notation to standard form, multiply the coefficient by 10 raised to the power of the exponent.
What are the rules for multiplying and dividing numbers in scientific notation?
When multiplying numbers in scientific notation, multiply the coefficients and add the exponents. When dividing, divide the coefficients and subtract the exponents.